Inline Block Example
Since the quality of pharmaceutical products affects human life, strict quality control is required not only during manufacturing, but also during transportation and storage to prevent deterioration. The standard aimed at ensuring the quality of pharmaceutical products during transportation and storage is the Good Distribution Practice (GDP).
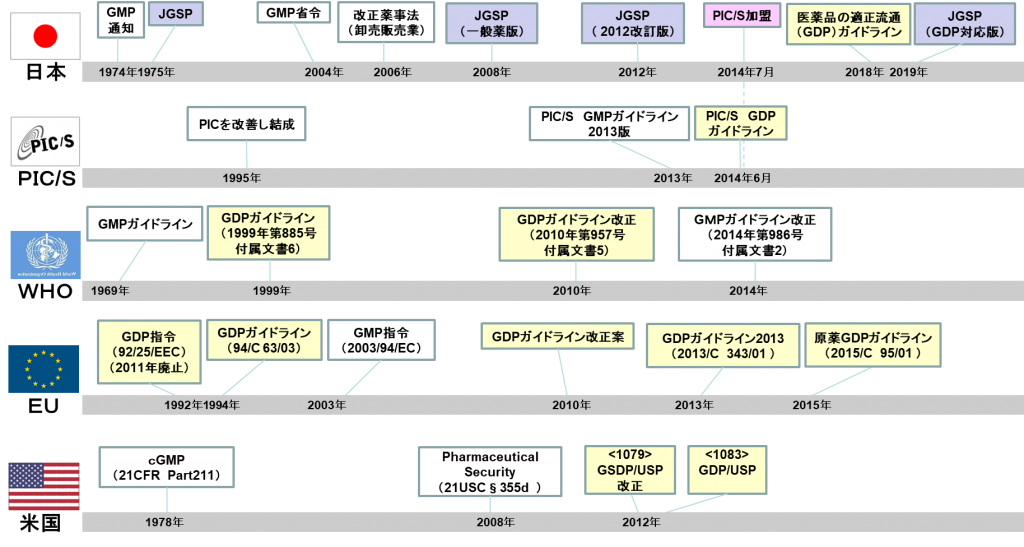
The World Health Organization (WHO) has GDP guidelines, and in the EU, GDP is a legal regulation. In Japan, the Ministry of Health, Labor and Welfare officially issued the GDP guidelines on December 28, 2018, as an administrative notice titled "
Guidelines for Good Distribution Practices (GDP) of Pharmaceuticals." In addition, the Japan Pharmaceutical Wholesalers Association (JGA) has reviewed the current JGSP, which is the association's voluntary standard, based on the Good Distribution Practices (GDP) guidelines, and has created the "JGSP (GDP International Harmonization Version)."
Regarding overseas pharmaceutical distribution standards
The WHO has had guidelines on pharmaceutical distribution since 1999, and in 2010 established the WHO good distribution practices for pharmaceutical products.
In the EU, the GDP directive was issued in 1992, before the WHO, and was significantly revised in 2013 to become the "Guidelines of 5 November 2013 on Good Distribution Practice of medicinal products for human use (2013/C 343/01)."
In the United States, the United States Pharmacopeial Convention (USPC) has established "<usp36 1079> Good Storage and Distribution Practices For Drug Products" and "<1083> Good Distribution Practices—Supply Chain Integrity."
Additionally, the PIC/S Pharmaceutical Inspection Convention and the Pharmaceutical Inspection Co-operation Scheme (PIC/S) also implemented GDP guidelines based on the EU GDP guidelines on June 1, 2014. One month later, in July 2014, Japan joined PIC/S, and so Japan's "
Good Distribution Practices (GDP) Guidelines" are now compliant with PIC/S and GDP.
Regarding overseas pharmaceutical distribution standards
Temperature control during pharmaceutical storage
The key temperature control requirements for pharmaceutical storage facilities in each GDP are as follows:
- Storage area temperatures are within acceptable limits
Validate that temperatures in storage areas such as warehouses are appropriate.
In particular, the temperature distribution Temperature Mapping Qualification will be performed by carrying out the following.
- Permanent temperature monitoring in appropriate locations within storage areas
Temperature monitoring in warehouses requires installing temperature sensors in appropriate locations according to the results of temperature mapping, and constantly monitoring the temperature and setting off alarms. The WHO/GDP recommends that the appropriate locations for installing temperature sensors should be prioritized in the hot spots and cold spots identified in temperature mapping, and seasonal variations should also be taken into consideration when deciding on locations where temperatures differ greatly between summer and winter.
- Alarm system when storage conditions are violated
A system is required that can quickly notification an alarm when the preset storage temperature conditions are exceeded.
- Implementing computerized system validation
Systems that use computers and monitoring and alarm systems will undergo computerized system validation. In order to handle temperature records and alarm history as electronic data, it will be necessary to comply with Part 11 and ER/ES pointer, which are requirements for electronic records and electronic signatures. In Japan's "Good Distribution Practice (GDP) Guidelines" Guidelines for proper management of computerized systems will be used as a reference.
- Periodic calibration of monitoring/alarm systems
The monitoring and alarm system must be periodically calibrated with a temperature calibration that is traceable to official standards such as national metrology standards. Calibration intervals must be determined based on risk and reliability assessments. At the same time, testing must be performed to ensure that the alarm system is functioning properly.
Our Response
We have equipment suitable for temperature control during the storage and transportation of pharmaceuticals and for the calibration of temperature control equipment, and can provide the optimal product depending on the size and purpose of the facility.
GDP also clearly defines requirements for temperature control of pharmaceuticals, as well as the maintenance, calibration, and inspection of equipment used in the storage and distribution of pharmaceuticals, and we are able to provide services to meet these requirements.
We also provide services such as temperature mapping and regular calibration, providing total support for temperature control in pharmaceutical distribution.
System Example
Applicable Products
One PC can centrally manage data from 360 transmitters. Up to six receivers can be connected via Ethernet, and each receiver can connect to up to 60 transmitters.
application software with Part 11-compliant security features is also available
Paperless recorder with 6 and 12 input points
Compact yet display with high performance, it is ideal as a standalone device or small-scale monitoring device.
When combined with the CISAS series, it can be configured as a recorder for a computer system.
Paperless recorder with up to 48 input points
With its versatile display and high performance, it is ideal for data management on devices such as stability testers, refrigerators, and freezers.
When combined with the CISAS series, it can be configured as a recorder for a computer system.
CISAS/V4 is a package system that uses our recorders, loggers, and controllers, as well as commercially available PLCs (programmable controllers), as system components to collect and monitor data from up to 5,000 tags of various devices and equipment on a PC.
Measurement data recorded by Part 11-compliant wireless loggers and graphic recorder can be centrally managed as electronic records.
Graph display by viewing data, daily reports, monthly reports, and reports can be created (printed and output as PDF files) and electronic signatures are possible
We are registration as a calibration laboratory for temperature and humidity under the Measurement Law.
We have also acquired certification as an "MRA-compliant certified business operator" based on ISO/IEC17025, and can issue calibration certificates with the JCSS certification symbol mark.
Our service technicians or contracted service personnel visit the user's site to inspect and calibrate measuring instruments, mainly temperature and humidity measuring instruments, regulators, recorders, etc.


