Inline Block Example
On December 28, 2018, the Ministry of Health, Labour and Welfare officially issued GDP guidelines in Japan in the form of an administrative notice entitled "Guidelines for Good Distribution Practice (GDP) of Pharmaceuticals."
The Good Distribution Practice (GDP) guidelines for pharmaceuticals are in accordance with the GDP of PIC/S (Pharmaceutical Inspection Convention and Pharmaceutical Inspection Co-operation Scheme) and reflect Japanese laws and regulations, ensuring international harmonization.
The essence of the Good Distribution Practice (GDP) guidelines for pharmaceuticals is
• Defines the method by which the integrity of medicinal products is maintained.
• Define appropriate measures to prevent counterfeit medicines from entering legitimate distribution channels.
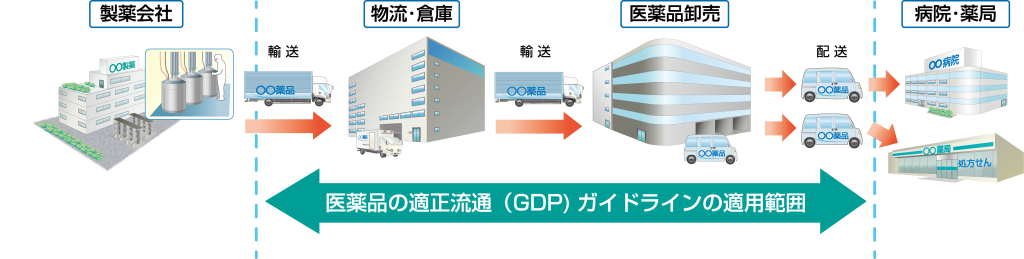
The scope of application is "the procurement, storage and supply of pharmaceuticals after they shipment onto the market, up until the time they are delivered to pharmacies, pharmaceutical sales businesses and medical institutions," and targets pharmaceuticals, excluding active pharmaceutical ingredients, cosmetics, replay medicine products, etc.
Temperature control of pharmaceuticals
Temperature control is important to maintain the integrity of pharmaceuticals, and consideration must be given to temperature control throughout all distribution routes from when pharmaceuticals are manufactured to when they reach patients. The Good Distribution Practice (GDP) guidelines for pharmaceuticals apply to the distribution route from transportation after shipment from pharmaceutical manufacturers (factories) to delivery to hospitals and pharmacies, and even within that scope, there are various distribution routes and forms, but in terms of operations, they are classified into storage and transportation, and the temperature control methods there are indicated in the guidelines.
The Good Distribution Practice (GDP) guidelines for pharmaceuticals configuration of a preface, objectives, scope, nine chapters, 40 items, and a glossary. Chapter 3: Facilities and Equipment and Chapter 9: Transportation contain information about temperature control.

Facilities, equipment and temperature control
The main items related to temperature control described in "Chapter 3 Facilities and Equipment" are summarized below.
- Facilities are kept clean, dry and within acceptable temperature ranges
- Consider the temperature, lighting, humidity and cleanliness of the facility, and establish procedure for environmental control and install equipment.
- Regardless of the size of the storage area, perform temperature mapping under appropriate conditions before use.
- Temperature monitoring equipment will be installed in appropriate locations according to the results of temperature mapping.
- Temperature mapping should be repeated if there is a change or major change in the storage environment
- Equipment used for control or monitoring should be regularly calibrated with a traceability to national metrology standards.
- Equipped with a system that issues an alarm when storage conditions are not met, and conducts regular inspections
- The computerized system will have security and electronic record protection functions and will be validated.
- The introduction and operation of computerized systems shall be based on the "Guidelines for Proper Management of Computerized Systems for Pharmaceutical and Quasi-Drug Manufacturers, etc."
Transportation and temperature control
The main items related to temperature control described in "Chapter 9 Transportation" are summarized below.
- Maintain acceptable temperature conditions during transport
- Investigate and report on temperature deviations during transportation
- Conduct risk assessment of transport routes to determine the need for temperature control
- Regularly maintain and calibrate equipment used to monitor temperatures in transport vehicles and containers
- The choice of shipping container or packaging should be based on the validation situation.
- Temperature control vehicles undergo temperature mapping under representative conditions
- Temperature control vehicles provide temperature monitoring during transport
- Use qualified temperature-control equipment and vehicles to transport temperature-sensitive medicines
- Establish procedure to control the transportation of temperature-sensitive medicines and seasonal temperature fluctuations
Our Response
We offer sensors, devices, systems, etc. suitable for temperature control and temperature control equipment in the storage, transportation, and distribution of pharmaceuticals, and can provide the optimal product depending on the size and purpose of the facility.
The Good Distribution Practice (GDP) guidelines also clarify requirements for temperature control of pharmaceuticals, as well as the maintenance and calibration of equipment that affects the storage and distribution of pharmaceuticals, and we are able to provide services to meet these requirements.
We also provide services such as temperature mapping, validation, and calibration, providing total support for temperature control in pharmaceutical distribution.
System Example
Applicable Products
One PC can centrally manage data from 360 transmitters. Up to six receivers can be connected via Ethernet, and each receiver can connect to up to 60 transmitters.
application software with Part 11-compliant security features is also available
Paperless recorder with 6 and 12 input points
Compact yet display with high performance, it is ideal as a standalone device or small-scale monitoring device.
When combined with the CISAS series, it can be configured as a recorder for a computer system.
Paperless recorder with up to 48 input points
With its versatile display and high performance, it is ideal for data management on devices such as stability testers, refrigerators, and freezers.
When combined with the CISAS series, it can be configured as a recorder for a computer system.
CISAS/V4 is a package system that uses our recorders, loggers, and controllers, as well as commercially available PLCs (programmable controllers), as system components to collect and monitor data from up to 5,000 tags of various devices and equipment on a PC.
Measurement data recorded by Part 11-compliant wireless loggers and graphic recorder can be centrally managed as electronic records.
Graph display by viewing data, daily reports, monthly reports, and reports can be created (printed and output as PDF files) and electronic signatures are possible
We are registration as a calibration laboratory for temperature and humidity under the Measurement Law.
We have also acquired certification as an "MRA-compliant certified business operator" based on ISO/IEC17025, and can issue calibration certificates with the JCSS certification symbol mark.
Our service technicians or contracted service personnel visit the user's site to inspect and calibrate measuring instruments, mainly temperature and humidity measuring instruments, regulators, recorders, etc.


