Atomic structure (electron arrangement)
Regarding the energy transitions of atomic nuclei and electron orbitals, we will first explain the atomic structure (electron arrangement).
As shown in the diagram below, there are electron orbitals around the nucleus. From the inside out, these electron orbitals are divided into types called the K shell, L shell, M shell, N shell, and so on, and the maximum number of electrons that can fit into each shell is fixed.
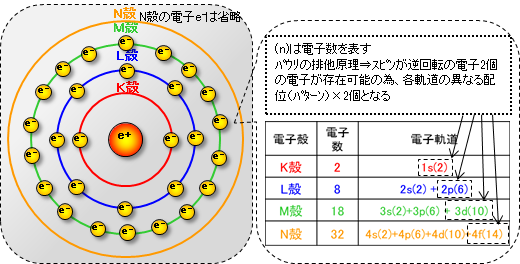
*The electron orbit is not actually a circular orbit as shown in the figure, but is more like a cloud that represents the high probability of an electron being at that level based on quantum mechanics, and the position of the electron itself cannot be determined.
Electron orbitals
In addition to electron shells, there are several different patterns of electron orbital shapes. These refer to the orbits (arrangement, distribution) in which electrons move around the nucleus, and are called electron orbitals, which are divided into several orbits as shown in the diagram above. (Schrödinger's wave equation)
s orbital;
There is one in each electron shell. Electrons are distributed in a spherical orbit around the nucleus (1s, 2s, 3s, ...). Up to two electrons can exist in one s orbital, even if the electrons rotation in opposite directions. (Pauli exclusion principle)
p orbitals;
Orbitals (2p, 3p, ...) exist in the L shell and above and are distributed in a figure-8 shape (gourd shape) around the nucleus. There are three orbitals of configuration in the X, Y, and Z axis directions, and up to six electrons can exist, including counter-rotation electrons.


